Amit Patel
SVP Medical
Safeguarding Patient Well-Being through Strategic Risk Management and Operational Excellence

A robust Pharmacovigilance (PV) & Safety system is essential for safeguarding patient well-being and maintaining regulatory compliance. SSI Strategy's PV & Safety team partners with you to provide strategic guidance and scientific expertise, helping you establish a safety system that prioritizes patient safety at every stage of your product's lifecycle.
By collaborating with our multidisciplinary team of medical and safety science experts, you gain access to deep therapeutic knowledge, regulatory insight, and operational excellence, ensuring your PV system is built on a foundation of quality, compliance, and patient-centricity.
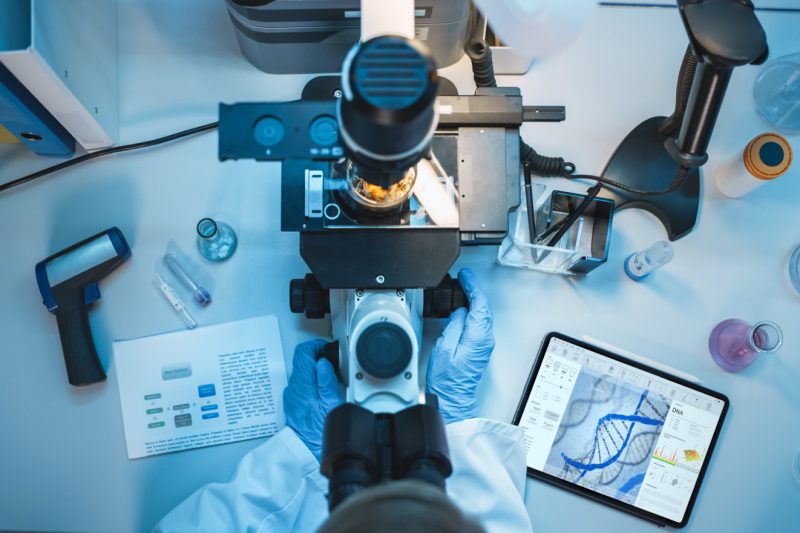
Effective risk management requires a comprehensive approach that integrates PV considerations into your product development and commercialization strategy. Our team collaborates with your functional stakeholders to develop a risk management plan that proactively identifies, assesses, and mitigates potential safety concerns. By leveraging our cross-functional expertise and data analytics capabilities, we help you design and implement risk minimization measures tailored to your product's unique safety profile and patient population.
This collaborative approach ensures that patient safety is prioritized throughout your organization, enhancing trust with regulators, healthcare providers, and patients.

Regulatory inspections are a critical test of your PV system's robustness and compliance. Our team partners with you to achieve inspection readiness, ensuring that your systems and documentation are prepared for regulatory scrutiny. We conduct thorough assessments to identify gaps and vulnerabilities, develop targeted remediation plans, and provide comprehensive inspection preparation support, including mock inspections and training.
By maintaining inspection readiness as an ongoing process, you can face regulatory challenges with confidence, demonstrating your commitment to patient safety and compliance.

Launching a new product successfully requires the integration of PV into your overall commercialization strategy. Our team collaborates with your cross-functional stakeholders to ensure that PV considerations are incorporated into your launch plan, from supply chain and labeling to medical affairs and marketing. By integrating PV into your launch strategy from the outset, you can minimize safety risks, optimize resource allocation, and accelerate time to market for your innovative therapy.
Our strategic partnership approach ensures that your PV launch strategy aligns with your global safety system, enabling a smooth transition from clinical development to post-marketing surveillance.

As your biotech company grows and your products advance through their lifecycle, your PV needs evolve. Our flexible, scalable support model allows us to adapt to your changing requirements, providing the expertise and resources you need at each stage of your journey. Whether you require end-to-end PV operations support, strategic guidance for signal detection and evaluation, or assistance with safety data management and reporting, our team provides the skills and experience you need to maintain an effective, compliant PV system.
By leveraging our agile approach, you can access top-tier PV expertise without the burden of maintaining a large internal infrastructure, enabling you to focus on your core mission of bringing innovative therapies to patients..

What distinguishes our offering is the ability to provide comprehensive support across all stages of your product's lifecycle, leveraging the diverse skill sets of our multidisciplinary team. From clinical development to post-marketing surveillance, our experts in PV, clinical operations, regulatory affairs, medical communications, and commercialization strategy work together to ensure that your PV system is fully integrated with your broader product development and go-to-market efforts.
By engaging with SSI Strategy, you gain access to a wealth of knowledge and experience that extends beyond the boundaries of a traditional PV service provider. Our flexible engagement model allows us to adapt to your evolving needs, providing the right mix of strategic guidance and hands-on support to help you navigate the complex biotech landscape with confidence.
SERVICE LEADERSHIP
Related Reading

OUR INSIGHTS
At SSI Strategy our experts stay abreast with the evolving environment and the new regulatory requirements that it gives rise to. It captures the state of the art of our industry today.
LET’S TALK
You don't have to go it alone!
Our experienced team has been there before, and we're ready to guide you through the unknown. Share your challenges with us, and together, we'll create a plan to efficiently reach your milestones and turn your vision into reality.
Building Better Biotechs