Laurie Smaldone Alsup
SVP Regulatory Science
Advanced strategies for clinical research and development

At SSI Strategy, we take a proactive, cross-functional approach to clinical development.
Our services include:
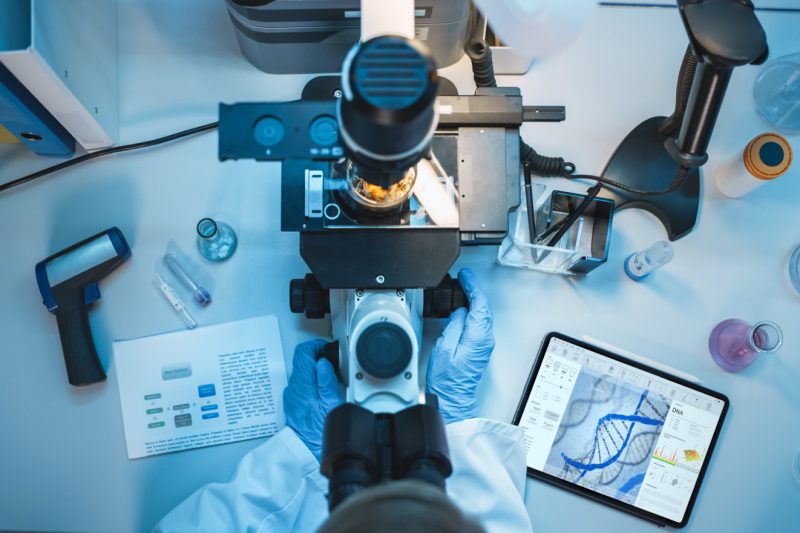
At SSI Strategy, we share your passion for bringing innovative new medicines to patients. We aim to be an extension of your team - a strategic thought partner providing the capabilities and bandwidth to translate your groundbreaking science into an approvable product.
From creating a compelling clinical development plan to executing your trials, we're here to support you every step of the way. Together, we'll maximize the probability of clinical and regulatory success for your program. Contact us to learn more about how we can assist with your clinical development needs.

SSI Strategy's clinical development team includes MDs, PhDs, and drug development professionals who have brought numerous innovative therapies successfully through the clinic to approval. We understand the nuances and challenges of clinical research, from early translational work to confirmatory pivotal trials.
Whether you are working on a complex rare disease program, a novel therapeutic modality, or navigating a unique regulatory pathway, we likely have encountered a similar scenario before. We apply our hard-earned insights to help you anticipate issues, make smart decisions, and achieve your development milestones.

Your clinical development needs ebb and flow throughout the product lifecycle. Rather than hiring full-time employees with specialized skill sets or relying on individual contractors, our agile resourcing model brings in the exact expertise you need, when you need it.
Our team will wrap around your existing resources to fill skill gaps, provide program management support, and execute your clinical development plan efficiently. As your program progresses and your requirements change, we flex to meet your evolving needs.

At SSI Strategy, we share your passion for bringing innovative new medicines to patients. We aim to be an extension of your team - a strategic thought partner providing the capabilities and bandwidth to translate your groundbreaking science into an approvable product.
From creating a compelling clinical development plan to executing your trials, we're here to support you every step of the way. Together, we'll maximize the probability of clinical and regulatory success for your program. Contact us to learn more about how we can assist with your clinical development needs.

SSI Strategy's clinical development team includes MDs, PhDs, and drug development professionals who have brought numerous innovative therapies successfully through the clinic to approval. We understand the nuances and challenges of clinical research, from early translational work to confirmatory pivotal trials.
Whether you are working on a complex rare disease program, a novel therapeutic modality, or navigating a unique regulatory pathway, we likely have encountered a similar scenario before. We apply our hard-earned insights to help you anticipate issues, make smart decisions, and achieve your development milestones.
SERVICE LEADERSHIP


SVP Regulatory Science


SVP Regulatory Science


SVP Regulatory Science


SVP Regulatory Science


SVP Regulatory Science


SVP Regulatory Science

OUR INSIGHTS
At SSI Strategy our experts stay abreast with the evolving environment and the new regulatory requirements that it gives rise to. It captures the state of the art of our industry today.
CONTACT
Contact us to learn how our experts can support you to meet all regulatory, quality and safety requirement and ensure the best path to approval.
Let’s Bring Medicines to the World.